The Cell-Mechanics lab in the Department of Applied Mechanics and Biomedical Engineering, IIT Madras, focuses on studying the mechanics behind health and disease, at cellular and sub-cellular scales. Borrowing upon principles from physical and mathematical sciences, we explore human biology that is responsible for, and is affected by, forces of interaction between mammalian cells and their surroundings.
 |
| Focus
areas of Cell-Mechanics lab: Metastatic extravasation and invasion,
Extracellular vesicles, Blood-cell assay development, Alveolar
transport, and Bio-materials development |
Some of the currently running research endeavors are in the field of (but not limited to):
- Cardio-oncology: Examining the cross-talk between hypertension therapy and metastasis.
- Approximating the cellular micro-environment: Recreating biophysical features specific to solid-tumor micro-environment, in experimental cell-culture model.
- Physics of cell-migration through extracellular matrices, and designing of efficient therapeutic schemes.
Related areas of interest include:
- Extravasation mechanics, for circulating tumor cells.
- Extracellular vesicles (EV): Characterization of secreted EVs during of pulmonary injury
- Biomaterials development: To expose cells to varying mechanical environments of matrix stiffness, architecture, and ligands.
- Visco-elasticity: of orthotropic collagenous scaffolds, plasma-membrane, extracellular-matrix, etc.
- Resistive pulse-sensing of suspended mammalian cells such as blood-cells.
Our collaborators include Physicists, Biologists, and Clinicians. Key skills that our research scholars develop are related to mammalian
cell-culture, numerous modes of light-microscopy, soft-lithography,
instrumentation and signal processing, bio-material synthesis and
mechanical characterization, and optics.
(i) Cardio-oncology:
 |
| Extravasation
involves an orchestrated dance of mechanics of cellular traction,
membrane, and endothelial junctional integrity, each affected by
cardiovascular drugs. |
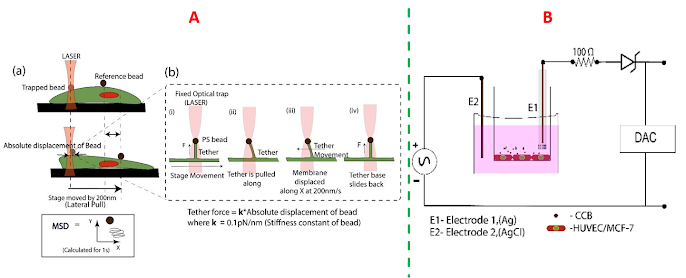 |
| Characterizing the membrane of endothelial cells, during hypertension-therapy, using optical tweezers (A), and endothelial integrity (B) using trans-endothelial electrical resistance (TEER) |
(ii) Designing cellular micro-environment
During
metastatic migration, cancer cells are exposed to a multiscalar
anistropic microenvironment. We are generating tunable engineered
microenvironments that allow control over the characteristic properties
of cell-microenvironment. In the micrograph (B, below), we see a highly disordered
cell-compatible matrix created through processing of silk-fibroin
protein, approximating the ordered architecture of ECM surrounding a solid-tumor (A, below)
 |
| Design of scaffolds for 3D models of cell-migration and growth, exhibiting micro-architecture that approximates the anisotropy around solid tumors. |
(iii) Physics of cell-migration
We are interested in quantifying the forces that a single or a cluster of cells exterts over a 2-D substrate in order to migrate effectively, and under varied physiological/pathological conditions. The micrograph shows a sample image from live-cell traction measurements we conducted for fibroblasts migrating over tunable 2-D substrates.
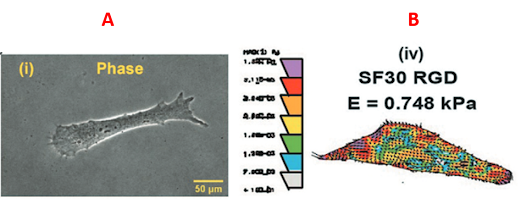 |
| We examine the traction force generated by cells, in both 2D and 3D, under various exogenous stimulatory cues. |
For more information, please feel free to contact us via. email, send in your tweet (@CellMech_IITM) or send us your query through the contact-us link on the top-right of this page.